A Design Qualification protocol is used at the stage where a design that has been developed from the VMP URS GAMP 5 cGMP and other Health and Safety Guidelines is reviewed and documented by competent persons to ensure that the designed equipment if built will satisfy all the detailed specified requirements as contained in the VP. All of the tests were performed and a report was generated.

Preparation Of Validation And Qualification Protocols Pharmaceutical Guidance
Approved Design Qualification Document.
. Qualification should be performed for new premises equipment. This HVAC Qualification or Validation protocol document. Test to be conducted while the room is at rest condition ie.
PURPOSE This test is to verify that the AHU dimensions position and sizes of utility connections are in compliance with the design qualification and also with as-built drawing. To design engineer and supply the Name of Equipment and to provide assurance that the machine is manufactured as per the URS. Risk based approach for HVAC qualification.
Finally the HVAC system was subjected to a performance qualification PQ study. Air Changes per Hour. Template for Design Qualification Protocol.
This protocol will be executed in compliance as per the requirements in 21CFR 210 211 ICH Q-7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients August 2001. 70 Equipment System Description 4 - 10. 20 Objective 3.
Page 2 of 27. On evaluation of the data collected during PQ it was found that the HVAC system met all the specified design criteria and complied with the entire cGMP requirement. PURPOSE This test is to verify that the AHU dimensions position and sizes of utility connections are in compliance with the design qualification and also with as-built drawing.
The Process User Requirements in the URS have been identified by the Quality Risk Assessment. DESIGN QUALIFICATION DQ PROTOCOL The DQ Protocol section of this qualification package defines and validates the Freezer System design. 30 Objective HVAC System Qualification Protocol.
The objective of this protocol is to provide an outline for the qualification of the HVAC system and to establish documentary evidence to demonstrate that the Air Handling Units AHUs are qualified to perform well within the predetermined acceptance criteria of performance as per. Clean rooms and associated controlled environments. 60 REFERENCES HVAC QUALIFICATION.
Design qualification should provide documented evidence that the design specifications were. The DQ Protocol section also defines and validates the manufacturer processes manufac-. Upon final approval of this Protocol and Summary Report the system.
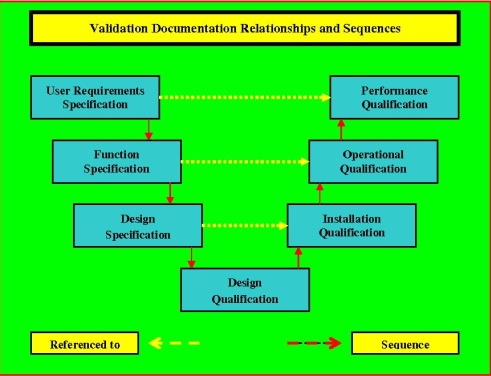
The main body is split into fourteen tables each one probing the design requirements and standards for the individual requirement. DQ is to verify that the system has been designed as specified in the URS User Requirements Specification FDS Functional Design Specification and relevant equipment specifications satisfying all GMP requirements. Aug 31 2015 Messages.
The Installation-Qualification is valid as documented evidence that the Air Handling Unit corresponds with all checkpoints of design plans all components of manufacturers documentation rules of technique and the defined requests The basis for the installation qualification was the detailed design room tables and the technical. PERFORMANCE QUALIFICATION PROTOCOL FOR HVAC SYSTEM OF AHU-01. To prove that each operation proceeds as per the design qualification and the tolerances prescribed there in the document.
Design Qualification Protocol HVAC. Design qualification process the design qualification process should address the following. 40 Responsibilities 3.
50 Pre-Requisites 3. The system will also be placed under formal change control in. SCOPE OF SUPPLY OF COMPONENTS.
Along with the attached Standard Operating Procedure will chapter by chapter take you through the task of raising fully detailed HVAC protocols. Heat Ventilation and Air conditioning. The goal of IQ is to verify and document the quality installation and integrity of HVAC system components.
VackerGlobal PO Box 92438 Deira Dubai United Arab Emirates. Validation of HVAC systems for design operation performance for storage and transportation of medicines and vaccines. Safety and security along with user.
SCOPE OF SUPPLY OF COMPONENTS. Fresh air filter G-4 Pre Filter G-4 F-5 Combo. B The premises supporting utilities and equipment have.
Full Guidelines on Validation in WHO TRS No 937 2006 Annex 4. Doc Number and it complies with the Scope of Supply. 60 References 4.
Qualification should be performed for new premises equipment utilities and systems at periodic intervals when major changes have been made. As built drawing 2. The result of particle count should comply with DESIGN QUALIFICATION.
30 Scope 3. 10 Design Qualification Protocol Approval 2. As built drawing 2.
Design validation of HVAC systems for storage and transportation of medicines and vaccines. OQ may be defined as. 70 ABBREVIATIONS AND DEFINITIONS.
Page 8 of 31 824 DQ IQ OQ PQ Protocol Mowden rev 0 V. INSTALLATION QUALIFICATION IQ 3. Documented verification that the system or subsystem performs as intended throughout all specified operating range.
No man power will be. Physically check the dimension of the ahu in length width and height and confirm with design document. To prepare detailed specifications design data for HVAC System and its major components to ensure that the User Requirement Specifications as.
During design qualification protocols which resulted in an ultimate pharmaceutical engineering design qualification is designed validation qualifications protocols for various zones. INSTALLATION QUALIFICATION IQ 3. This hvac qualification or validation protocol document.
This protocol will be performed utilizing 21 CFR 210 211 ICH Q-7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients August 2001. Become a Bronze Member and get products for free. Design documents and literatures are used to design installation protocols.
Calibration certificate of particle counter PROCEDURE TEST METHOD. Assisted system is ready for the essential part of performance and maintenance with system is conditioned and for hvac verification unless recorded and final. Template for Design Qualification Protocol OBJECTIVE To design engineer and supply the Name of Equipment and to provide assurance that the machine is manufactured as per the URS.
Qualification 1 Validation is an extensive exercise Qualification of the HVAC system is one component in the overall approach that covers premises systemsutilities equipment processes etc. Upon final approval of this IQ Protocol and Summary Report it will replace the previous IQ study and render it obsolete. Equipment and processes have been designed in accordance with the requirements for GMP Design Qualification.
Approved Design Qualification Document. DQ Design Qualification PURPOSE.
Design Qualification Fda Mhra Ema Who Validation Online
Hvac Operation Qualification Protocol

Design Qualification Dq Of Equipment Pharmaceutical Guidelines

Heating Ventilation And Air Conditioner Hvac Qualification

0 comments
Post a Comment